Is H2O polar or nonpolar and why? YouTube

Is Ch3Ch2Oh Polar Or Nonpolar? Understanding The Nature Of This
Expert-verified. Polarity of ethanol: ethanol is a polar molecule ( with dipole moment= 1.69)due to presence of hydro.. Name: Lab Partner: Lewis Structure (ethanol, CH3CH2OH) Date: Sketch - indicate polar bonds C - H ( H H Polar Bonds (Yes or No)? bo nd blo H 3c are nonPc lar H-o, c-O ave Polav Molecular Geometry at Carbon Atoms Molecular.

Best Overview Is CH3F Polar or Nonpolar Science Education and Tutorials
Answer: C2H6 (ethane) is a nonpolar molecule because it contains only nonpolar covalent bonds (C-H) bonds with both parts of the molecule cancelling out any small charge to ensure that there is no dipole moment. Is the word c2f2 polar or nonpolar? Home › Polar or Nonpolar › Is C2F2 ( Ethyne ) polar or nonpolar? Is C2F2 ( Ethyne ) polar or nonpolar?

Is oxygen gas polar or nonpolar? Gek Buzz
Polar vs Non-Polar molecules . As indicated in Table 2.6, the nature of molecular polarity determines the types of force(s) applied to a certain substance. So here we will have discussions about how to tell whether a molecule is polar or non-polar. The polarity of the compound can be determined by its formula and shape.

Solved 14) T he molecular geometry of the BeCl2 molecule is
Helium is nonpolar and by far the lightest, so it should have the lowest boiling point. Argon and N 2 O have very similar molar masses (40 and 44 g/mol, respectively), but N 2 O is polar while Ar is not. Consequently, N 2 O should have a higher boiling point. A C 60 molecule is nonpolar, but its molar mass is 720 g/mol, much greater than that.

Is CH3CH2OH Polar or Nonpolar? Polarity of Ethanol
Chemistry Chemistry questions and answers 4. Compound: CH3CH2OH (Ethanol) Lewis Structure 3-D Structure Skeletal Structure Functional Group (s) Present: Central Atom (s) Geometry: Polar or Non-Polar 5.

Is C2H5OH Polar or Nonpolar? (Ethanol) YouTube
H H ** ** ** H : C : C : O : H ** ** ** H H Q3. Give the electron pair and molecular geometries for each central atom. Q4. Give the bond polarity for each type of bond in the molecule. = There are two types of bonds in ethanol. The C-H bonds are nonpolar covalent. Q5. Is your molecule polar or nonpolar ? = Ethanol is both polar and nonpolar. Q6.
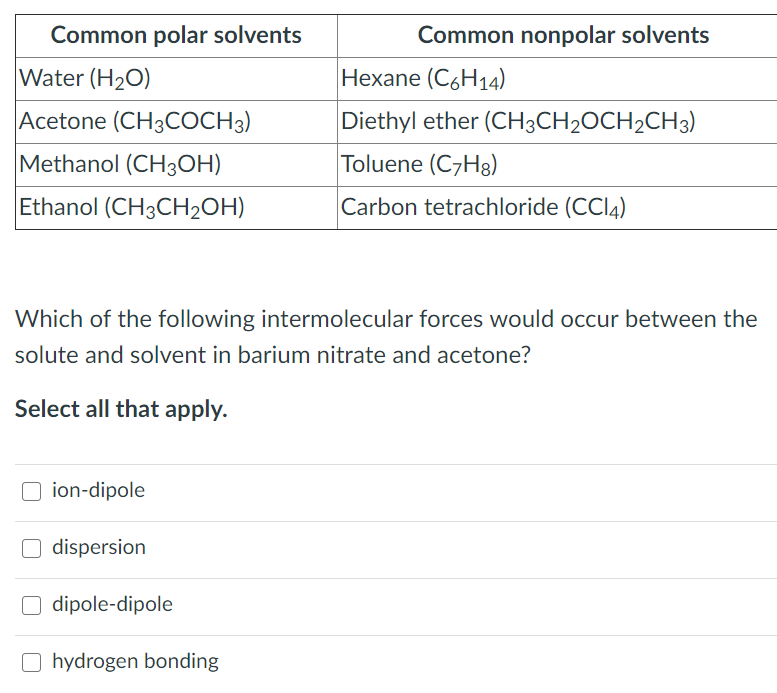
Solved Common polar solvents Water (H20) Acetone (CH3COCH3)
Learn to determine if CH3OH (Methanol) is polar or non-polar based on the Lewis Structure and the molecular geometry (shape).We start with the Lewis Structur.

science chemistry miscibility Fundamental Photographs The Art of
This answer is: Add your answer: Earn + 20 pts Q: Is ch3ch2oh polar or nonpolar Write your answer. Submit Still have questions? Find more answers Ask your question Continue Learning about.
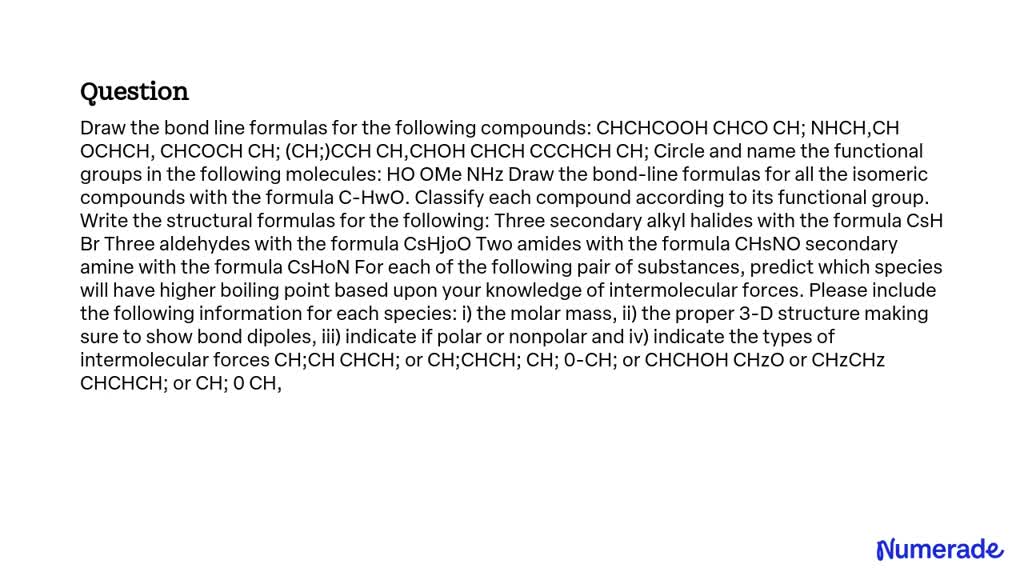
SOLVED Draw the bondline formulas for the following compounds
Chemistry can be a fascinating subject, especially when it comes to understanding the properties and behavior of different molecules. One such molecule that has been the subject of much discussion is CH3CH2OH. But the question is, is CH3CH2OH polar or nonpolar? Let's take a closer look to find out.Understanding Polar and Nonpolar MoleculesBefore we dive. Steel, an alloy of iron and carbon and small amounts of other metals, is an example of a solid solution. Table 6.3.1 6.3. 1 lists some common types of solutions, with examples of each. A solution is made by dissolving 1.00 g of sucrose ( C12H22O11 C 12 H 22 O 11) in 100.0 g of liquid water. Ethanol (CH3CH2OH) is an extremely polar chemical compound. Each CH3CH2OH molecule comprises two C-atoms, six H-atoms, and an O-atom. A small electronegativity difference (0.35 units) is present between a carbon and a hydrogen atom in each C-H bond. Exercise 2.12: Vitamins can be classified as water-soluble or fat-soluble (consider fat to be a very non-polar, hydrophobic 'solvent'. Decide on a classification for each of the vitamins shown below. Exercise 2.13: Both aniline and phenol are insoluble in pure water. Predict the solubility of these two compounds in 10% aqueous hydrochloric acid. The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. Calculate the electronegativity difference (ΔEN) and average ( EN) of the two electronegativities, and use the table below to determine the bond type and polarity. Helium is nonpolar and by far the lightest, so it should have the lowest boiling point. Argon and N 2 O have very similar molar masses (40 and 44 g/mol, respectively), but N 2 O is polar while Ar is not. Consequently, N 2 O should have a higher boiling point. A C 60 molecule is nonpolar, but its molar mass is 720 g/mol, much greater than that. 5. "Borderline" Polar Aprotic Solvents Have Small Dipole Moments And Low (<10) Dielectric Constants. These solvents have moderately higher dielectric constants than the nonpolar solvents (between 5 and 20). Since they have intermediate polarity they are good "general purpose" solvents for a wide range of reactions. Because non-polar solvents tend to be aprotic,the focus is upon polar solvents and their structures. Solvent Polarity. Solvents are generally classified by the polarity, and considered either polar or non-polar, as indicated by the dielectric constant. However, as with many properties, the polarity is a continuous scale, and the correct.POCl3 Lewis Structure (Phosphoryl Chloride) Lewis, Math, Molecules

Is O2 Polar Or Nonpolar?

Is H2O polar or nonpolar and why? YouTube

Best Explanation CH2Cl2 polar or nonpolar [N01] Science Education

draw the structure of ethanol molecule toctocquienes

Solved Name Lab Partner Lewis Structure (ethanol,

SOLVED CH3CH2OH has a much higher boiling * 3 points point than CH3O