NCl3 Polar or Nonpolar Nitrogen Trichloride Polarity Explained YouTube
MakeTheBrainHappy Is NCl3 Polar or Nonpolar?
Molecular Polarity. To determine if a molecule is polar or nonpolar, it is frequently useful to look at Lewis structures. Nonpolar compounds will be symmetric, meaning all of the sides around the central atom are identical - bonded to the same element with no unshared pairs of electrons.Polar molecules are asymmetric, either containing lone pairs of electrons on a central atom or having atoms.

Which is a nonpolar molecule H2CO HF NCl3 H2O C6H12 H2CO.docx
NCl3 Polar or Nonpolar: Nitrogen Trichloride Polarity Explained Geometry of Molecules 1.4K subscribers Subscribe 0 15 views 7 days ago Polarity of Molecules Today in this video, we are going to.

Brf3 Polar Or Nonpolar
1) is called a nonpolar covalent bond. Figure 4.4.1 4.4. 1 Polar versus Nonpolar Covalent Bonds. (a) The electrons in the covalent bond are equally shared by both hydrogen atoms. This is a nonpolar covalent bond. (b) The fluorine atom attracts the electrons in the bond more than the hydrogen atom does, leading to an imbalance in the electron.

Solved 4. Predict the molecular shape of each of the
NCl3 is the chemical formula for Nitrogen trichloride. Also, called trichloramine it is a halogen nitride that is yellow and oily with a pungent smell. It is known as a strong explosive because being unstable in the pure form and sensitive to heat, shock, light, and any organic compound.
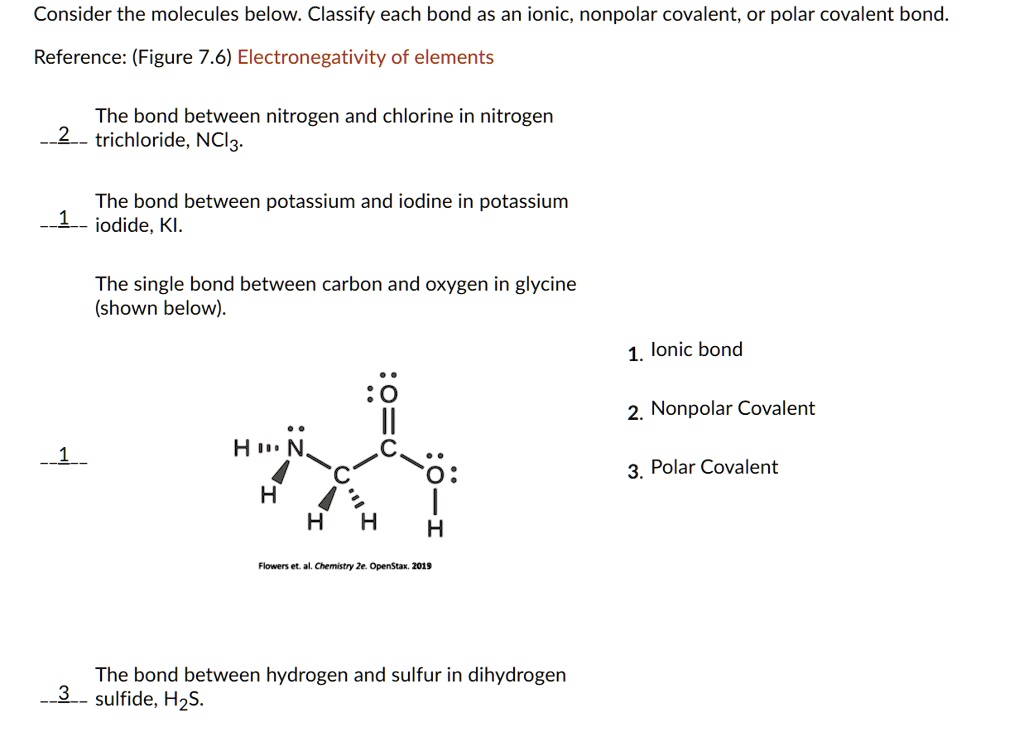
SOLVED Consider the molecules below Classify each bond as an ionic
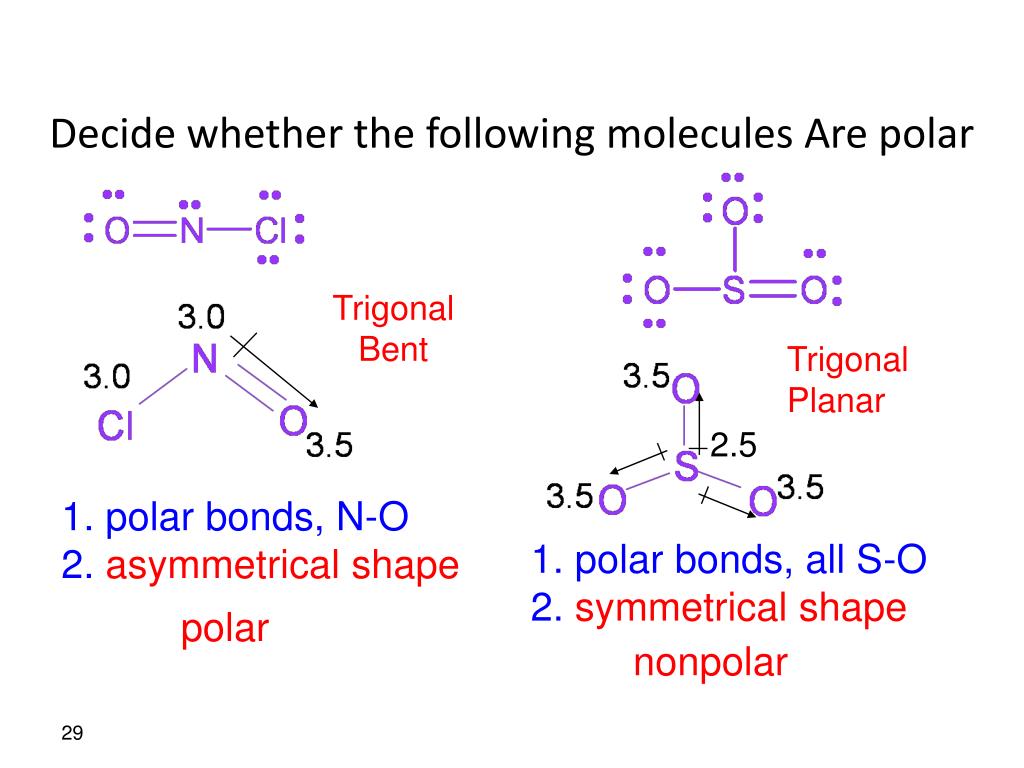
Draw Lewis structures, name shapes and indicate polar or non-polar for the following molecules: a. CH 4 b. NCl 3 c. CCl 2 F 2 d. CF 2 H 2 e. CH 2 O f. CHN g. PI 3 h. N 2 O i. SO 2 j. CS 2 k. CO l. H 2 O m. COF 2 n. N 2 o. O 2 p. H 2 q. Cl 2 r. HF s. O 3 t. NI 3. a. CH 4 tetrahedral, non-polar b. NCl 3 trigonal pyramidal, polar c. CCl 2 F 2.

Is NCl3 Polar or Nonpolar? Techiescientist
Is NCl3 Polar or Nonpolar? Answer: NCl3 is a polar molecule due the presence of a lone pair of electrons. This leads to electron-electron repulsion which results in a bent structure, thereby causing an unequal distribution of charge within the molecule and creating a permanent dipole.

Solved ose the selection which correctly characterizes all
Explain. Question: Is N C l 3 polar or nonpolar? Explain. Nitrogen Trichloride: Nitrogen trichloride ( N C l 3) is an organic compound that used to be a bleaching agent for bread flour. Safer.

Ch4 Polar Or Nonpolar / Solved How Many Nonbonding Electrons Are In Ch4
Learn to determine if NCl3 (Nitrogen trichloride) is polar or non-polar based on the Lewis Structure and the molecular geometry (shape).We start with the Lew.

Is NCl3 (Nitrogen trichloride) Polar or NonPolar? YouTube
Notice that a tetrahedral molecule such as CCl4 CCl 4 is nonpolar Figure ( 4.12.1 4.12. 1. Another non polar molecule shown below is boron trifluoride, BF 3. BF 3 is a trigonal planar molecule and all three peripheral atoms are the same. Figure 4.12.1 4.12. 1 Some examples of nonpolar molecules based on molecular geometry (BF 3 and CCl 4 ).

NCl3 Polar or Nonpolar Nitrogen Trichloride Polarity Explained YouTube
NCl3 is a slightly polar molecule because of the small difference between the electronegativity of nitrogen and chlorine atom. NCl3 molecule has one lone pair that leads to repulsion between electrons and the shape of the molecule is trional pyramidal. Nitrogen trichloride is a yellow oily liquid with its pungent odor.
PPT Modern Chemistry Chapter 6 Chemical Bonding PowerPoint
Nitrogen trichloride is a very explosive substance that appears like an oily liquid with the chemical formula NCl3. It smells similar to chlorine. It has a dipole moment of 0.6 D that shows it is moderately polar.

sobre " Ncl3 " e polar ou apolar? existe nessa molécula 3 ligações
(And Why?) May 27, 2023 by Jay Rana NCl3 is a covalent (polar covalent) compound because when one nonmetal combines with another nonmetal, it usually forms a covalent compound. Here, N is a nonmetal and Cl is also a nonmetal. So when they combine, it forms a covalent compound.

NCl3 Part 3 Polarity YouTube
NCl3 is a slightly polar molecule. This is because nitrogen has a lone pair of electrons that repels the bonded electron pairs of the N-Cl covalent bonds, thus giving the molecule an asymmetric structure where the polarities of the bonds do not cancel each other out. Nitrogen and chlorine have similar electronegativities, making individual N-Cl.
Lewis Structure Ch4 Polar Or Nonpolar PPT CHM 2045 Molecular
nonpolar covalent: electronegativity difference is less than 0.4 (nonmetal+nonmetal close together on the periodic table) polar covalent: electronegativity difference in between 0.4 and 2.0 (nonmetal + nonmental further apart on the periodic table) ionic: electronegativity difference is above 2.0 (metal + nonmetal)

Polar & Nonpolar Covalent Bonds YouTube
Nitrogen trichloride (NCl3) is a weakly polar molecule. The central nitrogen (N) atom in the NCl3 molecule is surrounded by three chlorine (Cl) atoms via single covalent bonds, forming an asymmetric trigonal pyramidal shape. The electronegativity of the chlorine (Cl) atom is slightly higher than the nitrogen (N) atom.
SOLVED VSEPR Structure and Shape of Molecules CO2 SO3 NCl3 Crude
Want to know the reason? Let's dive into it! NCl3 is a POLAR molecule because it has a lone pair of electrons on the Nitrogen atom (N) which causes the entire molecule to bend. This bending of NCl3 molecule results in asymmetric geometry, which makes the molecule polar.