Electron Configuration Of Oxygen In Ground State
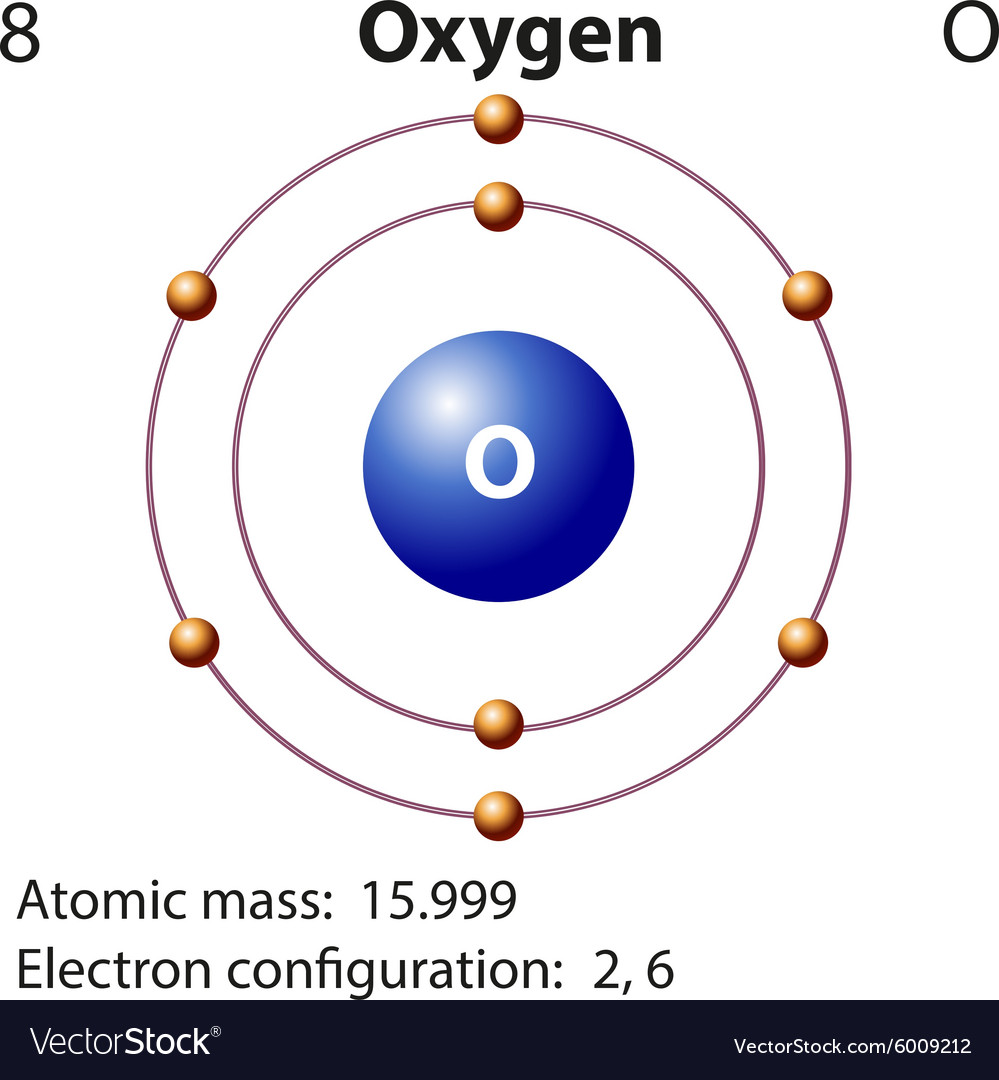
Diagram representation element oxygen Royalty Free Vector
Oxygen is a chemical element with atomic number 8 which means there are 8 protons and 8 electrons in the atomic structure.The chemical symbol for Oxygen is O. Electron Configuration and Oxidation States of Oxygen. Electron configuration of Oxygen is [He] 2s2 2p4. Possible oxidation states are -2. Electron Configuration
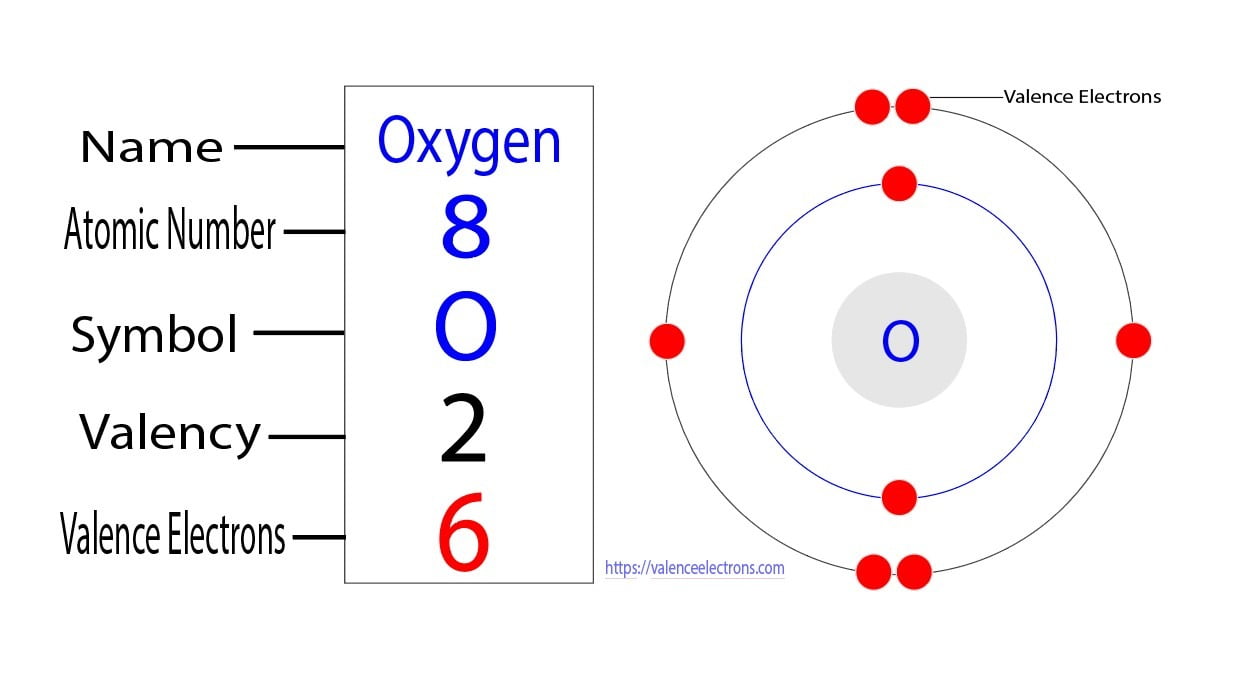
How to Find the Valence Electrons for Oxygen (O)?
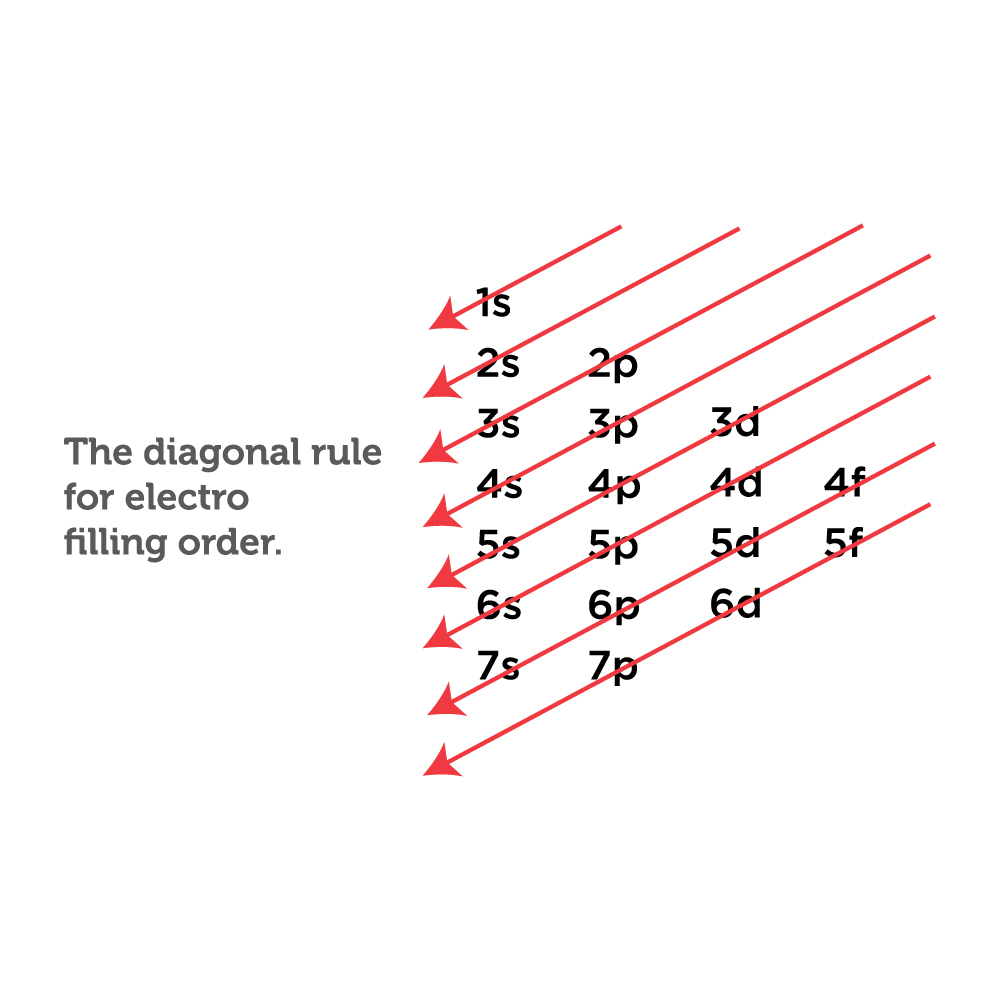
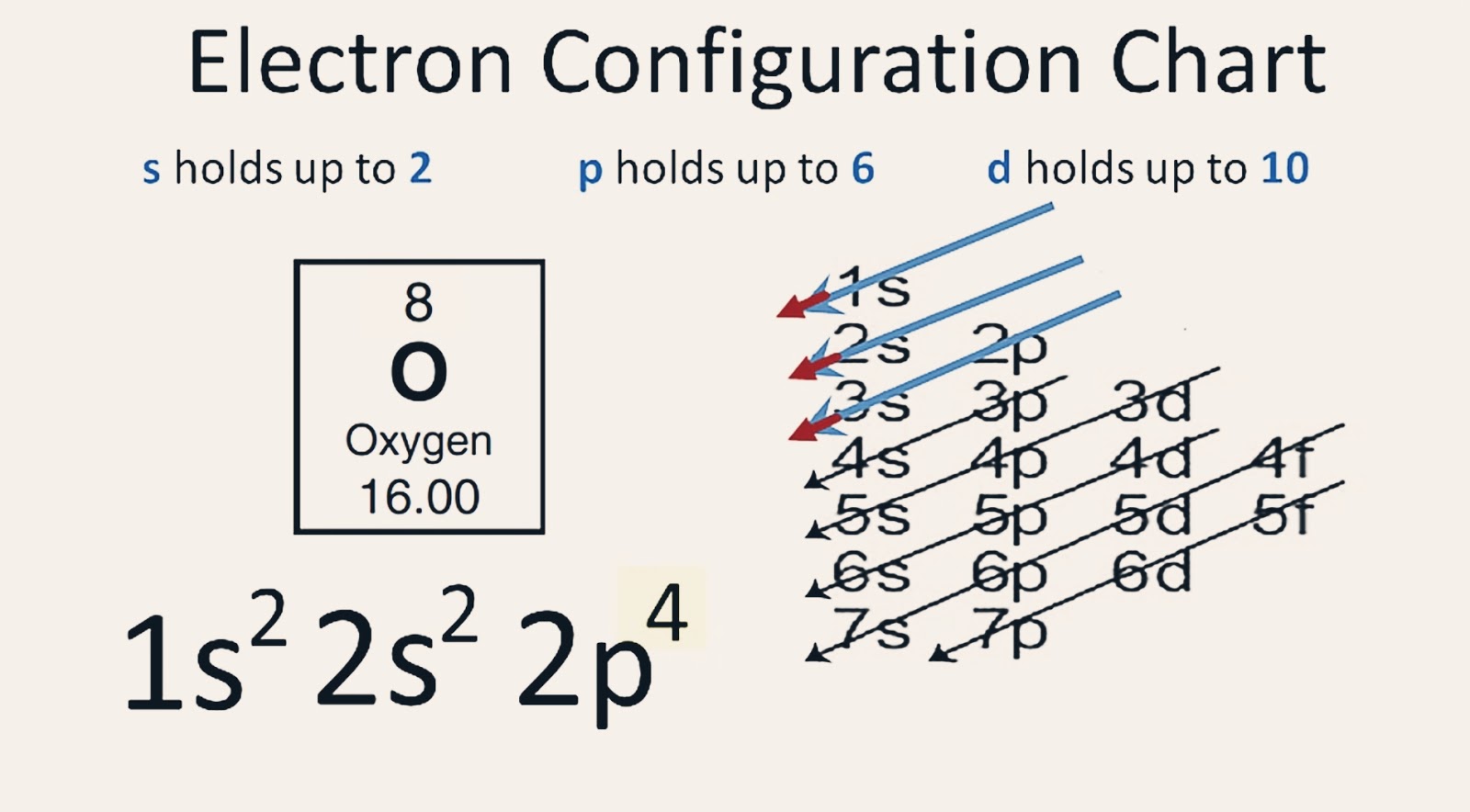
Based on the order of fill above, these 8 electrons would fill in the following order 1s, 2s and then 2p. So Oxygen's electron configuration would be O 1s 2 2s 2 2p 4. Special Cases. Configurations of ions present a special case of electron configuration and also demonstrate the reason for the formation of those ions in the first place.
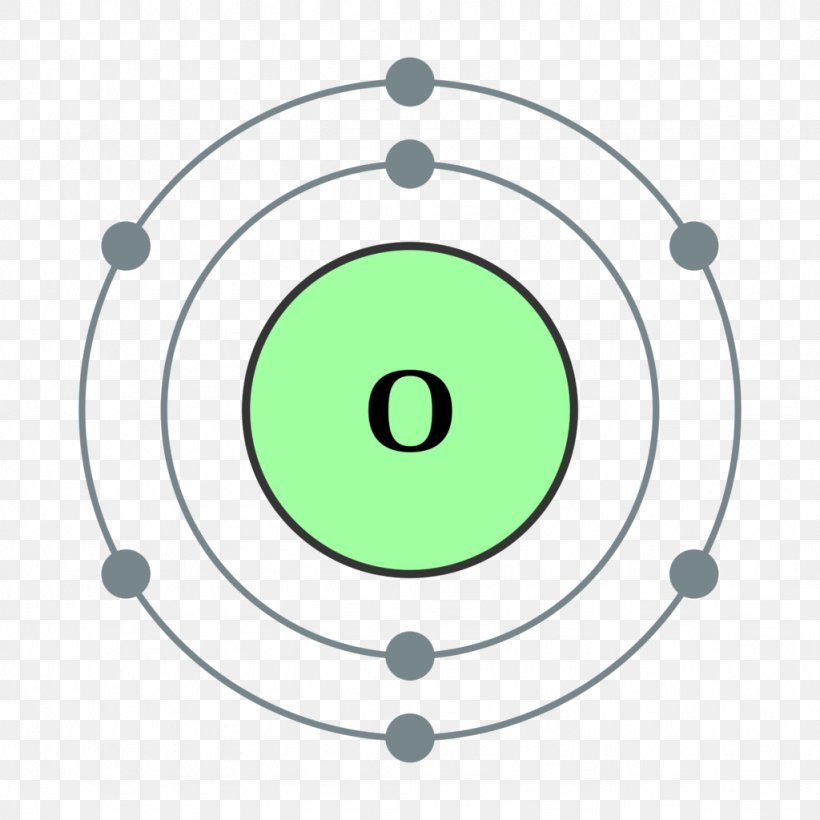
Atomic Number Oxygen Bohr Model Chemical Element, PNG, 1024x1024px
Introduction. The electron configuration is the standard notation used to describe the electronic structure of an atom. Under the orbital approximation, we let each electron occupy an orbital, which can be solved by a single wavefunction. In doing so, we obtain three quantum numbers (n,l,m l), which are the same as the ones obtained from solving the Schrödinger's equation for Bohr's hydrogen.

【5 Steps】Oxygen Electron Configuration in Just 5 Steps Electron
Let's find the electron configuration of Oxygen! A single oxygen atom has 8 protons and 8 electrons, but how do we know where Oxygen puts its electrons, in w.

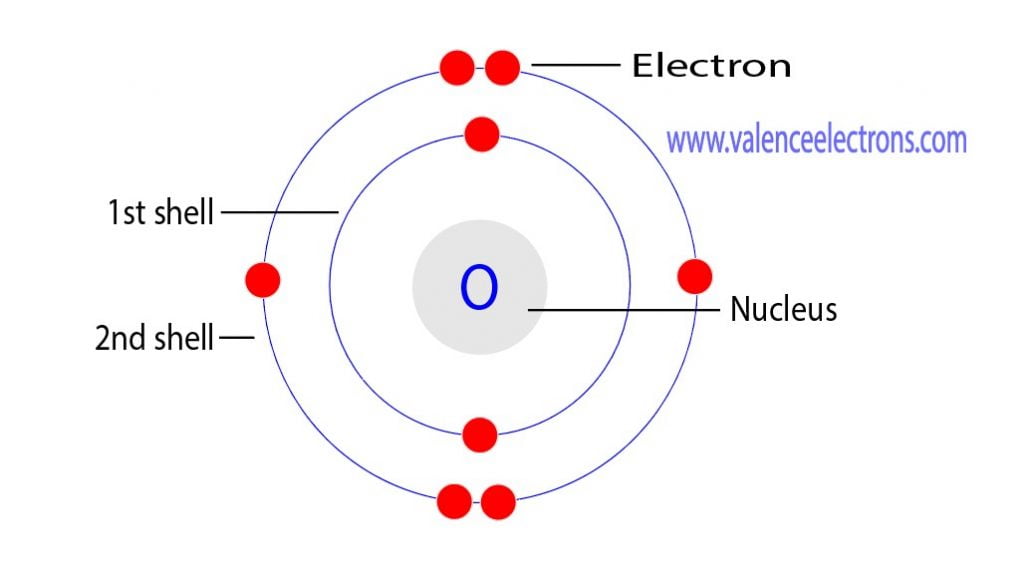
Oxygen Bohr Model (Diagram, Steps To Draw) Techiescientist
The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. We describe an electron configuration with a symbol that contains three pieces of information ( Figure 6.25 ): The number of the principal quantum shell, n,
How to Write Ground State Electron Configuration in Chemistry
The arrangement of electrons in oxygen in specific rules in different orbits and orbitals is called the electron configuration of oxygen. The electron configuration of oxygen is [ He] 2s 2 2p 4, if the electron arrangement is through orbitals. Electron configuration can be done in two ways. Electron configuration through orbit (Bohr principle)

Electron Configuration Of Oxygen In Ground State
In this case, 2+2+6+2+6+2+10+6+2+1= 39 and Z=39, so the answer is correct. A slightly more complicated example is the electron configuration of bismuth (symbolized Bi, with Z = 83). The periodic table gives the following electron configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p65s2 4d10 5p6 6s2 4f14 5d10 6p3.

Electron Configuration for Oxygen (O, O2 ion)
The electron configuration of an oxygen atom [He] 2s 2 2p 4 suggests that neutral oxygen atoms can achieve an octet of valence electrons by sharing two pairs of electrons to form an O=O double bond, as shown in the figure below. According to this Lewis structure, all of the electrons in the O 2 molecule are paired.

The electron configuration of oxygen is 1s2,2s2 2p4. Science
This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation.
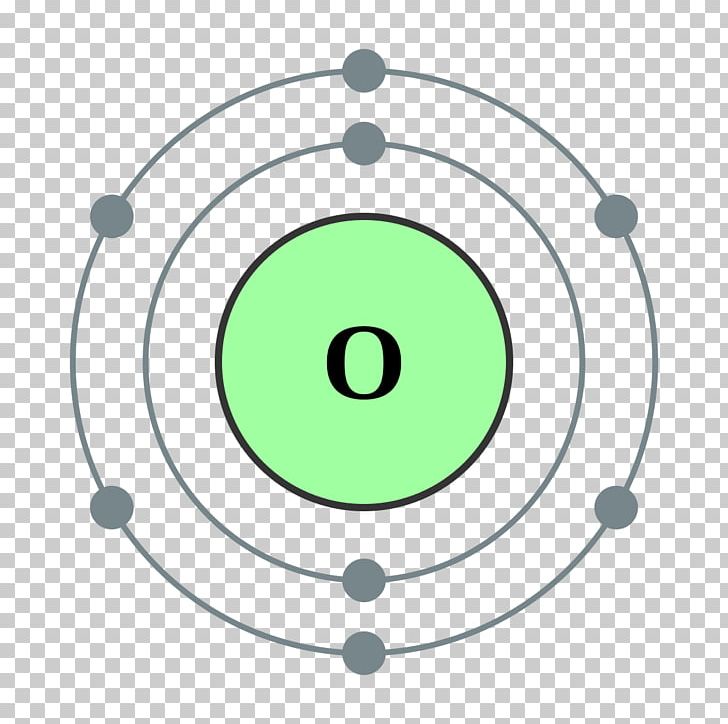
Bohr Model Chemical Element Oxygen Atomic Theory PNG, Clipart, Angle
Electronic configuration: The arrangement of electrons into the orbitals of an atom using some fundamental principle is called its electronic configuration. Electronic configuration of Oxygen: The electronic configuration of Oxygen is 1 s 2 2 s 2 2 p 4. Oxygen requires two electrons to attain noble gas configuration. Suggest Corrections 24
What Is the Oxygen Electron Configuration(O)?
Electron configurations are a simple way of writing down the locations of all of the electrons in an atom. As we know, the positively-charged protons in the nucleus of an atom tend to attract negatively-charged electrons.

What is the Electron Configuration of Oxygen Archives Dynamic
Ground State Electron Configuration of Oxygen. The way electrons are arranged in oxygen is shown by the numbers 1s^2, 2s^2, 2p^4. This tells us how many electrons are in each part. Let's break it down and explain it more simply. Oxygen has eight electrons. The first energy level can hold two electrons, and oxygen has two at this level.
Oxygen(O) electron configuration and orbital diagram (2022)
Wayne Breslyn 728K subscribers Join Subscribe Subscribed 799 130K views 4 years ago In this video we will write the electron configuration for O 2-, the Oxide ion. We'll also look at why Oxygen.
Oxygen Electron Configuration (O) with Orbital Diagram
Electron Configuration Notation: -shows the arrangment of electrons around the nucleus of an atom. - helps chemist understanding how elements form chemical bonds. - can be written using the period table or an electron configuration chart. How to Write the Electron Configuration for Oxygen Oxygen is the eighth element with a total of 8 electrons.

Symbol and electron diagram for Oxygen Royalty Free Vector
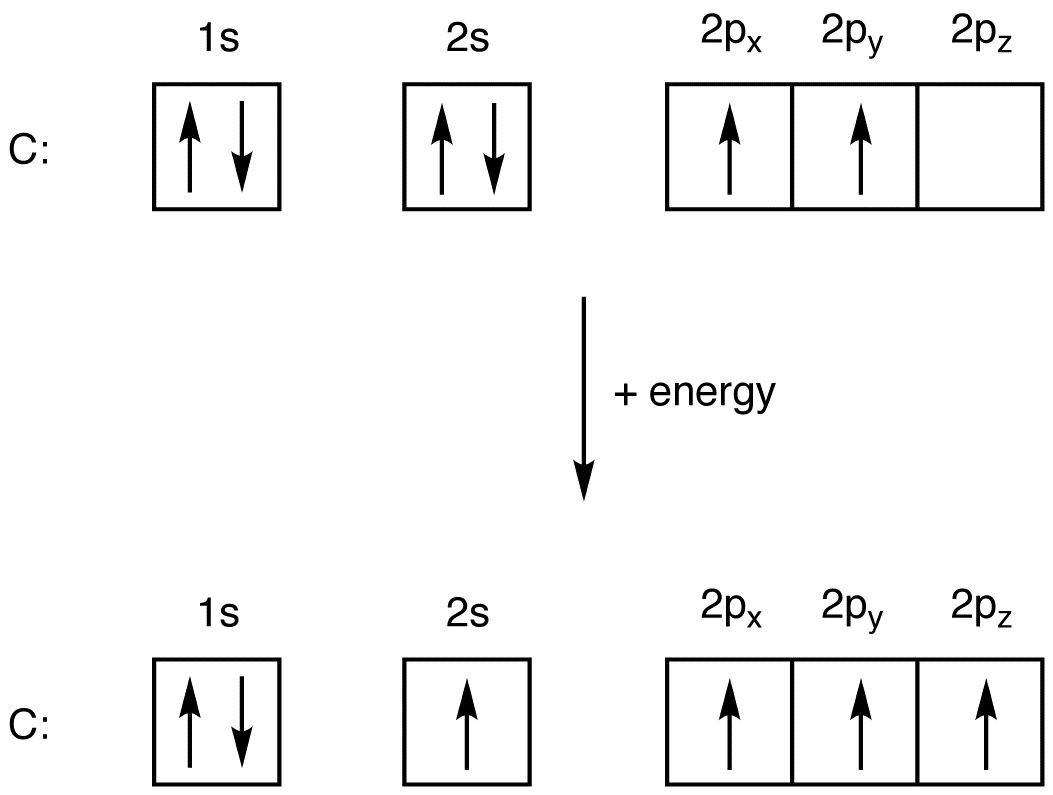
The first two electrons in lithium fill the 1 s orbital and have the same sets of four quantum numbers as the two electrons in helium. The remaining electron must occupy the orbital of next lowest energy, the 2 s orbital (Figure 8.3. 3 or 8.3. 4 ). Thus, the electron configuration and orbital diagram of lithium are:

Oxygen Atom Science Notes and Projects
If we look at the element after nitrogen in the same period, oxygen (Z = 8) its electron configuration is: 1s 2 2s 2 2p 4 (for an atom). Oxygen has one more electron than nitrogen and as the orbitals are all half filled the electron must pair up. Occupation of Orbitals.