SO3 Lewis Structure How to Draw the Lewis Structure for SO3 (Sulfur Trioxide) YouTube

Lewis Dot Structure for SO3 (Sulfur trioxide) YouTube
A step-by-step explanation of how to draw the SO3 Lewis Dot Structure (Sulfur trioxide).For the SO3 structure use the periodic table to find the total number.

Sulfur trioxide Alchetron, The Free Social Encyclopedia
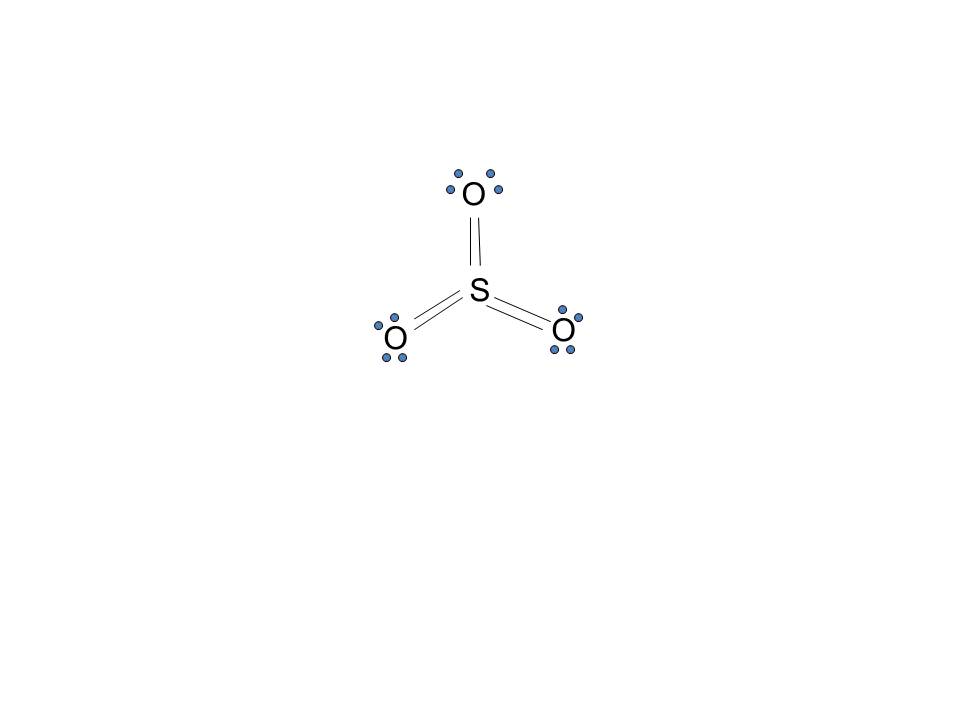
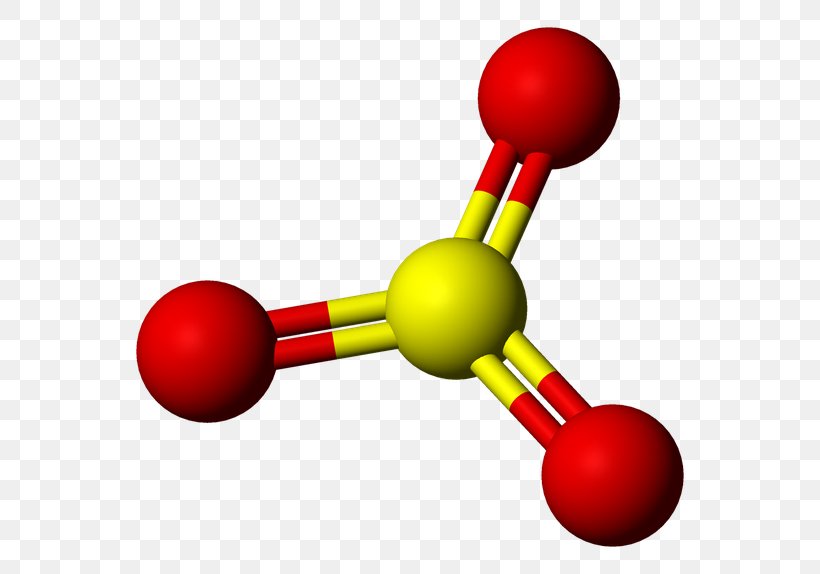
Lewis structure of SO3 The sulfur trioxide is a tetra atomic chemical molecule where both the sulfur and three oxygen molecules bond with an equal number of valence electrons. The diagram is drawn showing dots of valence electrons around the symbol of both sulfur and oxygen atoms with lines predicting bond formation.
Sulfur Trioxide Sulfur Dioxide Lewis Structure Oleum, PNG, 1200x1254px, Sulfur Trioxide, Area
This chemistry video explains how to draw the Lewis structure of SO3 - Sulfur Trioxide. It discusses the molecular geometry, bond angle, hybridization, and.
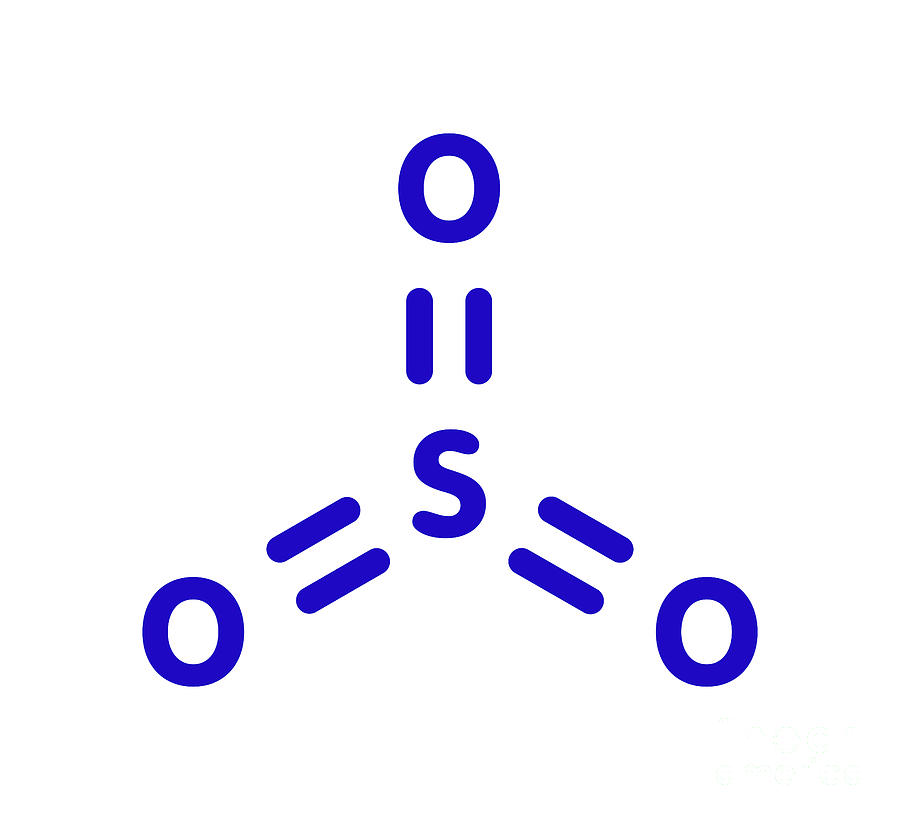
Sulfur Trioxide Pollutant Molecule Photograph by Molekuul/science Photo Library
1.3K 357K views 10 years ago SO3 Lewis, Shape, Hybridization, Polarity, and more. A step-by-step explanation of how to draw the SO3 Lewis Dot Structure (Sulfur trioxide). For the SO3.

SO3 Lewis Structure How to Draw the Lewis Structure for SO3 (Sulfur Trioxide) YouTube
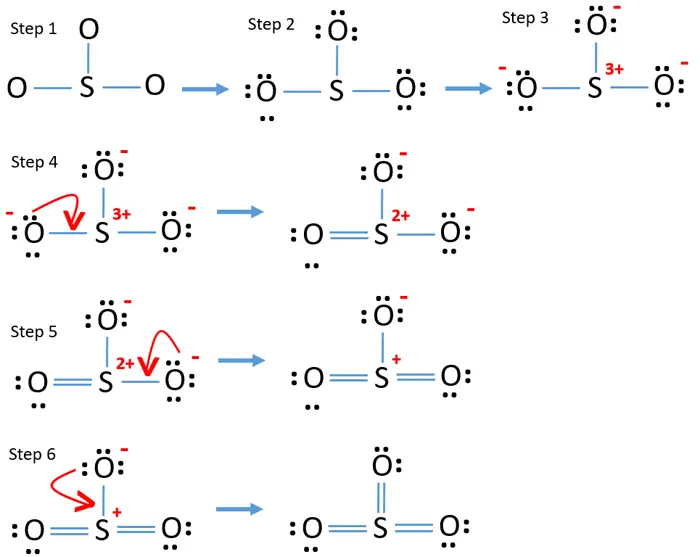
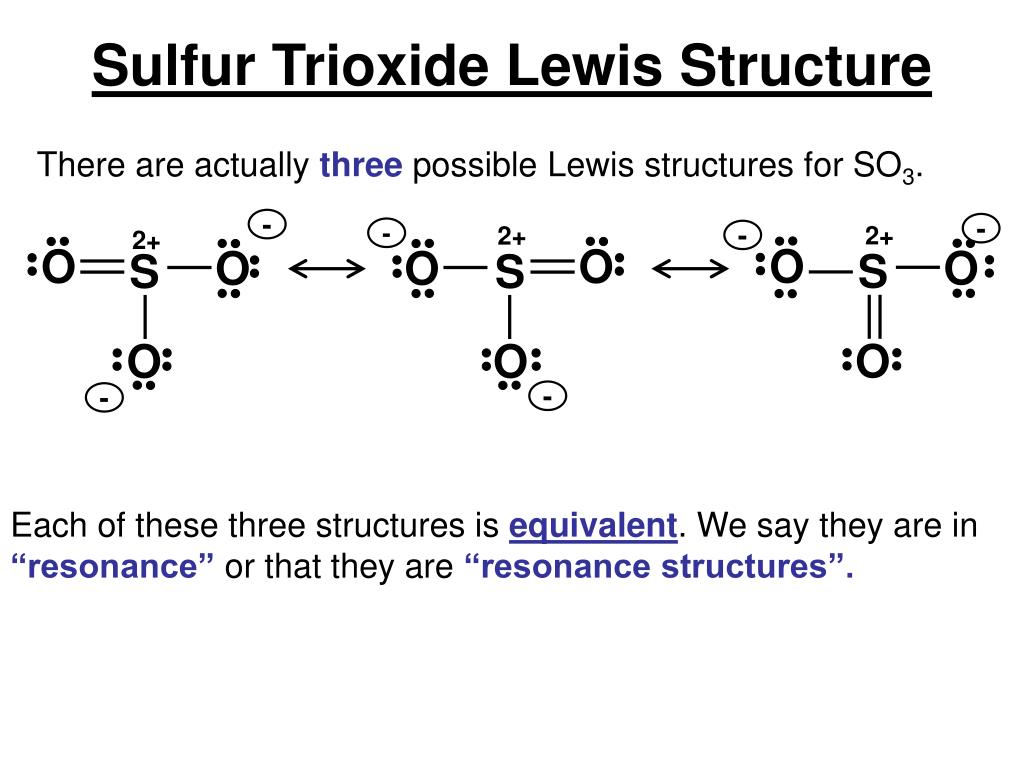
Lewis Structure of SO3 Valence: Here, sulfur in the center because of its lowest electron capability, and three oxygen around it. Sulfur brings 6, and oxygen brings 3 each. That means; SO3 has 24 valence electrons. 6 + (3 x 6) = 24. Now have a look of Lewis Structure again; When we draw it, firstly we get the three structures at the top.
Lewis Dot Diagram For Sulfur Wiring Site Resource
Chemistry Chemistry questions and answers Draw the Lewis structure for the sulfur trioxide (SO_3) molecule. Be sure to include all resonance structures that satisfy the octet rule. This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer

Draw The Lewis Dot Structure For So3 2 slidesharedocs
Subscribed 628K views 9 years ago Lewis Structures How to draw the Lewis Structure of SO3 (sulfur trioxide) - with explanation Sulfur is an exception to the octet rule - it can handle up.

SO3 Lewis Structure, Molecular Geometry, and Hybridization Techiescientist
Watch on Steps of drawing SO3 lewis structure Step 1: Find the total valence electrons in SO3 molecule In order to find the total valence electrons in SO3 (sulfur trioxide) molecule, first of all you should know the valence electrons present in sulfur atom as well as oxygen atom.
39 lewis dot diagram for so3 Wiring Diagram
A video explanation of how to draw the Lewis Dot Structure for Sulfur Trioxide, along with information about the compound including Formal Charges, Polarity, Hybrid Orbitals, Shape, and.

So3 2 Lewis Structure
Lewis Dot of Sulfur Trioxide. SO 3. Back: 70 More Lewis Dot Structures. S does not follow the octet rule. It will hold more than 8 electrons. Sulfur having valence electrons in the 3rd energy level, would also have access to the 3d sublevel, thus allowing for more than 8 electrons. NOT IN THIS MOLECULE.
IC1 Repaso de Examen de Química
Lewis structure of SO 3 (or Sulfur trioxide) contains three double bonds between the Sulfur (S) atom and each Oxygen (O) atom. The Sulfur atom (S) is at the center, surrounded by 3 Oxygen atoms (O). The Sulfur atom does not have a lone pair, while all three Oxygen atoms have 2 lone pairs.

So3 sulfur trioxide molecule Royalty Free Vector Image
We draw Lewis Structures to predict: -the shape of a molecule. -the reactivity of a molecule and how it might interact with other molecules. -the physical properties of a molecule such as boiling point, surface tension, etc. Drawing the Lewis Structure for SO 3 ( Sulfur Trioxide) SO 3 is the primary contributer to acid rain in the atomsphere.

Free download Sulfur trioxide Sulfur dioxide Lewis structure Chemistry, text, logo png PNGEgg
The SO3 Lewis structure shows a central Sulfur (S) atom with three Oxygen (O) atoms around it. These atoms are connected by double bonds, and each Oxygen atom has two lone pairs of electrons. In this page, you'll find a detailed, step-by-step guide on how to draw the Lewis structure for SO3. Step-by-Step Guide to Drawing the Lewis Structure of SO3
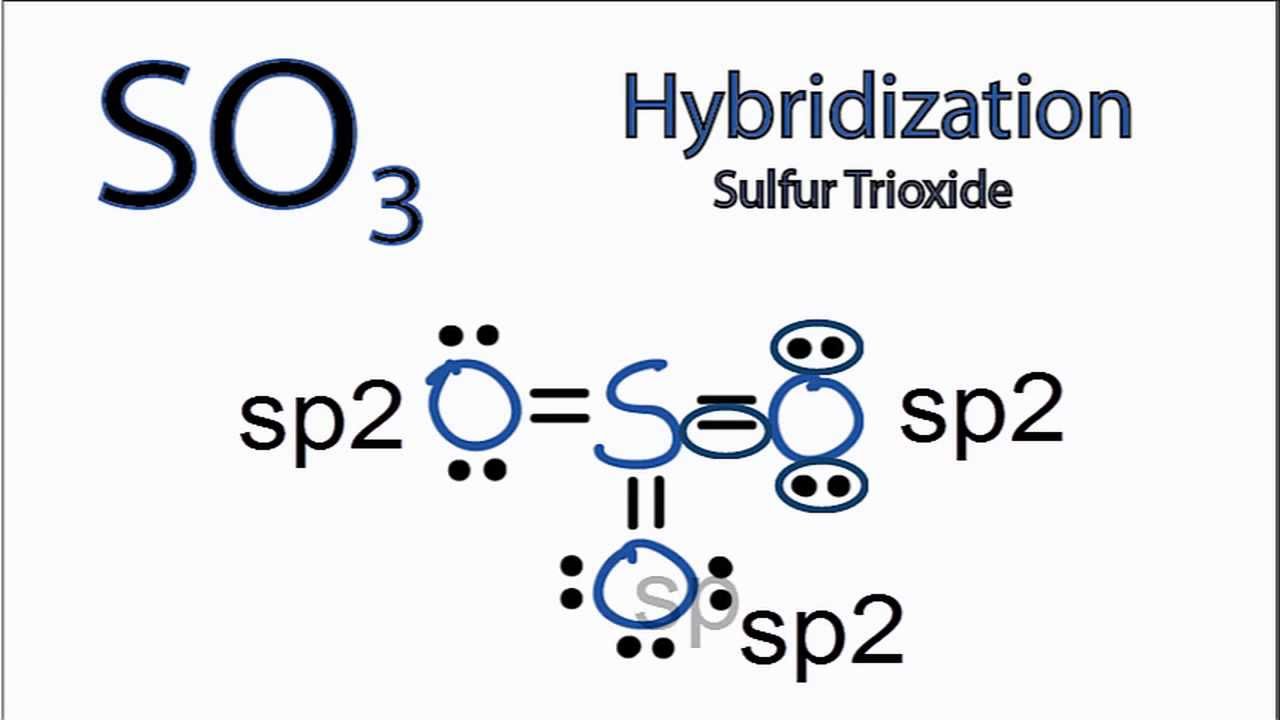
SO3 Hybridization Hybrid Orbitals for SO3 (sulfur trioxide) YouTube
Sulphur trioxide has three resonance structures. But all the structures are equivalent because all the oxygen atoms in SO3 are equivalent. In sulphur trioxide, one sulphur atom and three oxygen atoms are present. In SO 3, sulphur is sp 2 hybridized with a trigonal planar structure and bond angle is 120 0.
PPT Chapter 8 PowerPoint Presentation, free download ID6001268
The SO3 Lewis structure illustrates how the atoms of sulfur trioxide, a molecule composed of one sulfur atom and three oxygen atoms, are arranged. Within the SO 3 Lewis structure, the sulfur atom is bonded to three oxygen atoms through double bonds. Additionally, each oxygen atom has two lone pairs of electrons associated with it.
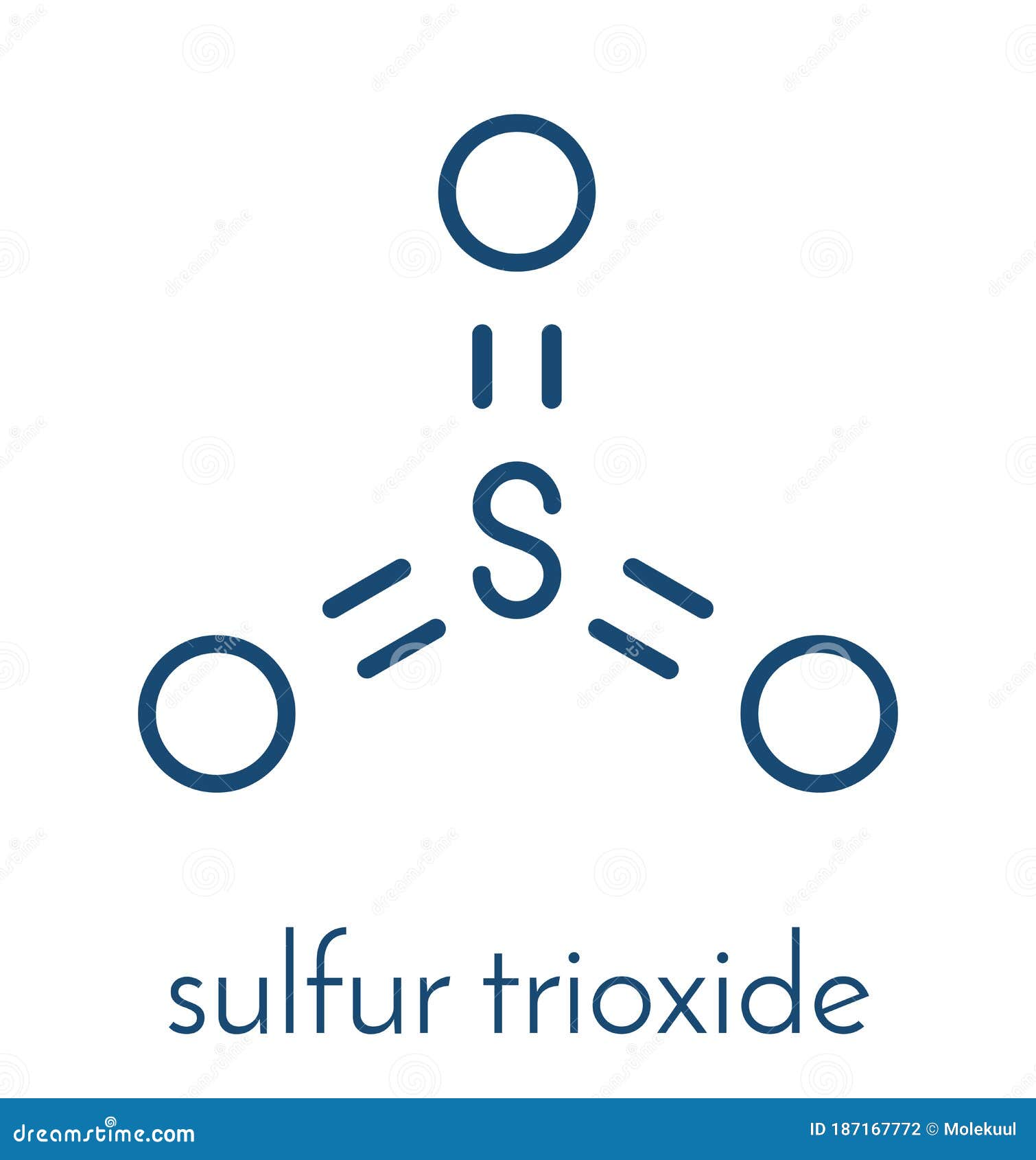
Sulfur Trioxide Pollutant Molecule. Principal Agent In Acid Rain. Skeletal Formula. Vector
2. The problem is that Lewis structures very rarely give a realistic account of the actual bonding situation in a molecule. It is rather good for organic molecules but for molecules like SO3 it is not suitable. Fact is that all S − O-bonds in SO3 are the same and they are neither typical single nor double bonds.